Inter-laboratory,Validation,of,Real-time,PCR,Assays,for,Goat-,,Horse-,and,Donkey-derived,Material,in,Meat,Products
Qiang WANG Yicun CAI Xue NING Litao YANG Zhenjian XU Lina ZHAO Liangwen PAN


Abstract In this study, we performed an inter-laboratory collaborative ring trial to develop and validate specific TaqMan real-time PCR assays for goat-, horse-, and donkey-derived material in meat products. The performances of these assays in different environments and situations were comprehensively evaluated. This ring trial involved the participation of 12 laboratories in Europe and Asia. The results from the participating laboratories were analyzed to determine the specificity, accuracy, false positive rate, limit of detection (LOD), and probability of detection (POD) of the developed assays. Statistical analysis showed that the false positive and negative rates were zero, the LOD was five copies/reaction, and the laboratory standard deviation (σL) was 0.30 for all three assays. Thus, the results demonstrate that the developed methods are robust and suitable for the detection and identification of goat-, horse-, and donkey-derived materials in meat products.
Key words Real-time PCR; Food and feedstuff industry; Collaborative trial; Adulteration; Species identification
Received: December 21, 2020 Accepted: February 21, 2021
Supported by National Key Research and Development Program of China (2017YFC1601700); Shanghai Science and Technology Commission Standard Special Fund (19DZ2205000); Shanghai Science and Technology Commission Technology Platform Research Fund (20DZ2291900).
Qiang WANG (1966-), male, P. R. China, researcher, devoted to research about molecular biological detection technology.
*Corresponding author. E-mail: panlw888@126.com.
The identification and quantification of animal materials derived from different species in food and feedstuffs are indispensable for ensuring food safety. Additionally, incidences of food adulteration with goat-, horse-, or donkey-derived materials attract significant public attention and can cause problems in various social and religious contexts[1-2]. For example, recent scandals involving horse meat in processed meals and supposedly halal beef burgers adulterated with pork caused widespread shock and distress[3-4]. Accordingly, many countries and regions have strict regulations that require food and feedstuffs to be labelled with accurate and detailed information regarding the identity of all animal materials therein[5]. Biological surveillance of the adulteration of animal products is a major challenge for governmental agencies[6]. However, there are very few widely acknowledged and recognized International Organization for Standardization (ISO) standards for the detection of animal materials in food and feedstuffs. Consequently, establishing recognized international standards for the identification of animal materials in food and feedstuffs is urgently required to ensure human health and international trade safety.
Techniques that rely on DNA detection are most commonly used to identify the source of animal materials due to its high stability and ubiquity in tissues[7-8]. Furthermore, the DNA-based methods used in species identification, such as PCR and real-time PCR, have been demonstrated to be fast, reliable, and sensitive[9], and such methods are typically easy to operate one-step procedures with good specificity, high accuracy, high efficiency, high sensitivity, and a low false-positive rate[9-14]. Accordingly, numerous real time PCR methods have been developed and applied to the identification of biological materials from specific animal species.
In this study, species-specific TaqMan real-time PCR assays for the detection of goat-, horse-, and donkey-derived components in meat products were developed and validated, and their performances were comprehensively evaluated through an international collaborative ring trial.
Materials and Methods
Meat materials and DNA extraction
Reference genome DNAs of bison (Bison bison), cat (Felis catus), camel (Camelus bactrianus), cattle (Bos taurus), chicken (Gallus gallus), dog (Canis familiaris), duck (Anas platyrhynchos), elk (Cervus canadensis), goat (Capra hircus), horse (Equus caballus), human (Homo sapiens), mouse (Mus musculus), ostrich (Struthio camelus), pig (Sus scrofa domesticus), rabbit (Oryctolagus cuniculus), rhesus macaque (Macaca mulatta), sheep (Ovis aries), rat (Rattus norvegicus) and turkey (Meleagris gallopavo), were purchased from Zyagen Laboratories (San Diego, CA, USA). Blood samples of several breeds of donkey (Equus asinus), goat (Capra hircus), goose (Anser anser), hinny (Equus caballus ♂ × Equus asinus ♀), horse (E. caballus) and mule (E. caballus ♀ × E. asinus ♂) were obtained from local slaughterhouses (Jiujiang, Jiangxi; Jining, Shandong; Jiaxiang, Shandong; Yili, Xinjiang; Luan, Anhui, China) and the entire slaughtering process was carefully monitored. DNA samples from several breeds of donkey were obtained from Northwest Sci-Tech University of Agriculture and Forestry (Yangling, China). Water buffalo (Bubalus bubalis) DNA was obtained from Huazhong Agricultural University (Wuhan, China). Hair samples from Przewalskis horse (Equus przewalskii) and zebra (Equus burchellii) were obtained from Shanghai Zoo. Indian zebu (Bos indicus) blood samples were obtained from the Academy of Grassland and Animal Science (Yunnan, China). Certain plant samples, DNA samples from several horse breeds, and other animal samples were obtained from Shanghai Customs.
The animal hair (cleaned with water) and other solid samples were milled into superfine powder in liquid nitrogen using a Freezer Mixer (6850 Freezer/Mill, SPEX Sample Prep, USA), then used for DNA extraction and detection procedures.
Genome DNA was extracted using a published phenol/chloroform extraction method[15] (ISO 21571:2005). Briefly, 100 mg of meat powder sample was mixed with 800 μl of extraction buffer and 20 μl of proteinase K solution (20 mg/ml, Tiangen, China). After incubation at 65 ℃ for 60 min with occasional vigorous shaking, an equal volume of phenol/chloroform/isoamyl alcohol (25∶24∶1, v/v) was added to each sample. The samples were then thoroughly mixed and centrifuged at 5 000×g for 15 min. The resulting supernatant was collected and mixed with an equal volume of chloroform/isoamyl alcohol before centrifugation at 5 000×g for 10 min. The aqueous layer was transferred to a clean tube and 2.5 volumes of ice-cold 96% ethanol and 0.1 volume of 3 mol/L potassium acetate (pH 5.2) were added. Samples were mixed, incubated at -20 ℃ for 30 min, and centrifuged at 5 000×g for 30 min. The supernatant was discarded and the pellet was washed twice with 800 μl of 70% ethanol. Following centrifugation at 5 000×g for 15 min, the pellet was air-dried and re-suspended in 100 μl of DNAse- and RNAse-free water (Invitrogen, USA). The DNA concentration was measured using a NanoVue spectrophotometer (GE Healthcare, UK).
Recombinant Plasmid Construction
A recombinant pUC57-ghd plasmid (Sangon Biotech, Shanghai, China) containing goat-, horse-, and donkey-specific DNA fragments was constructed (Fig. 1) and used as a calibrator to determinate the limit of detection (LOD) and probability of detection (POD) values of the real-time PCR assays. Sequencing results of the pUC57-ghd plasmid confirmed single-copy insertion of each species-specific DNA fragment without deletion or insertion mutations (Fig. 2). The absolute copy number of the plasmid pUC57-ghd was determined by analyzing the three target DNA fragments using a Bio-Rad QX200 droplet digital PCR system (Hercules, CA, USA).
Primers and probes
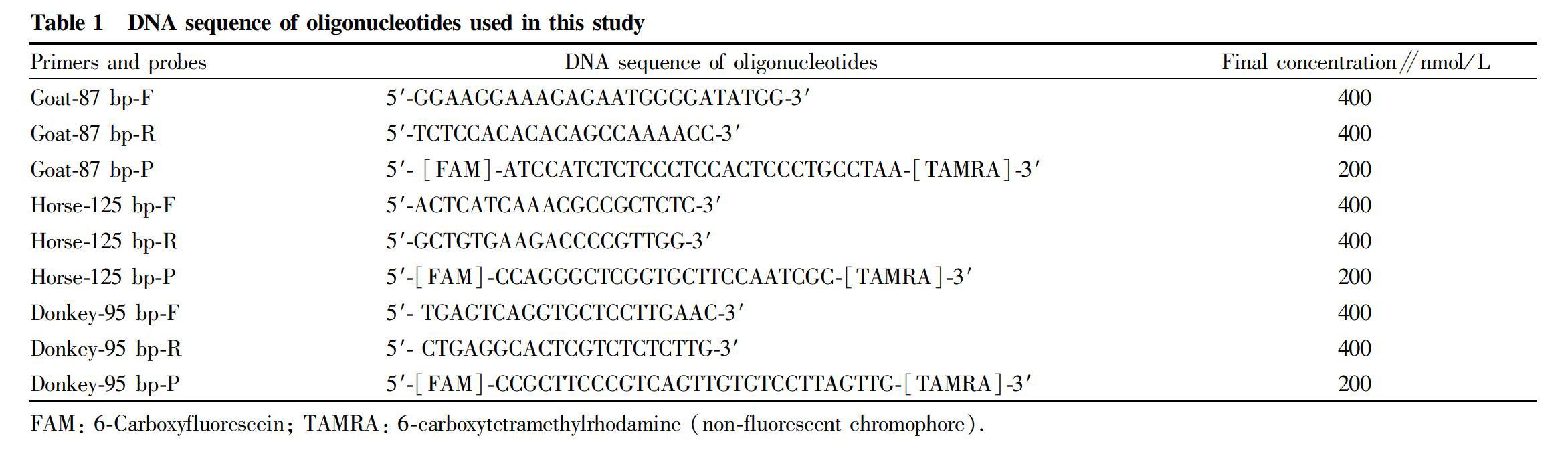
For the detection of goat-, horse-, and donkey-specific material, the goat (Capra hircus) chromosome 9 gene (GenBank accession number: NC_030816.1)[16], horse (Equus caballus) chromosome 28 gene (GenBank accession number: NC_009171.2)[17], and donkey (E. asinus) genomic scaffold gene (GenBank accession number: NW_014638576.1)[18] were selected as species-specific target sequences. Primers and TaqMan probes were designed using Primer Express Software version 3.0 (Foster City, CA, USA) and are listed in Table 1. The specificity and homology of the primers and probes were evaluated by BLASTN searches against the entire GenBank database.
Real-time PCR
Real-time PCR was performed with a 25 μl reaction volume comprising primers 1 μl each (10 μmol/L), probe (10 μmol/L) 0.5 μl, real-time PCR Master Mix (Bio-Rad, Hercules, CA, USA) 12.5 μl, nuclease- and protease-free water (Thermo Scientific, Salt Lake City, UT, USA) 5 μl, and sample DNA or plasmid DNA 5 μl. For amplification, different real-time PCR instruments and corresponding software versions (ABI7500 Fast v 2.3, ABI ViiA7 v 1.2, ABI7900HT v 2.3, Bio-Rad CFX96 v 3.1, Quant Studio TM12K Flex v 1.2.2 and Roche Light Cycler 480 v 1.5) were used in the collaborative ring trial. The thermal cycling program included an initial denaturation step at 95 ℃ for 10 min followed by 45 cycles of 15 s at 95 ℃ for denaturation and 60 s at 60 ℃ for annealing and extension. Fluorescence signals were recorded in the extension step of each cycle.
Specificity evaluation of real-time PCR assays
Specificity, including exclusivity and inclusivity, was evaluated according to established practices[16]. To assess exclusivity, DNA samples from goat, horse, donkey, and 24 non-target animal species were used. The inclusivities for goat, horse, and donkey DNA were tested using several breeding lines representing each species. The concentration of the reference DNA samples used in the exclusivity and inclusivity tests was 20 ng/μl. In addition, the Sanger DNA sequencing method[19] was utilized to determine the species identity of all reference DNA samples used in this work, enabling us to rule out heterogeneous contamination and guarantee the reliable specificity of the test results.
Qiang WANG et al. Inter-laboratory Validation of Real-time PCR Assays for Goat-, Horse- and Donkey-derived Material in Meat Products
Collaborative ring trial
The collaborative ring trial was organized by the Technical Center for Animal, Plant, and Food Inspection and Quarantine, Shanghai Customs. A total of twelve laboratories from five countries (China, France, Germany, Malaysia, and Portugal) participated in the collaborative trial. Each laboratory received a package including the following samples and reaction reagents: ① 12 DNA samples with a volume of 50 μl (A1-A12, sample codes randomly assigned) for goat-specific detection, including six tubes of goat genomic DNA samples (2 copies/μl) and six tubes of bovine genomic DNA samples (4 copies/μl), ② 12 DNA samples with a volume of 50 μl (B1-B12, sample codes randomly assigned) for horse-specific detection, including six tubes of horse genomic DNA samples (2 copies/μl) and six tubes of bovine genomic DNA samples (4 copies/μl), ③ 12 DNA samples with a volume of 50 μl (C1-C12, sample codes randomly assigned) for donkey-specific detection, including six tubes of donkey genomic DNA samples (2 copies/μl) and six tubes of bovine genomic DNA samples (4 copies/μl), ④ a pUC57-ghd plasmid DNA sample with a volume of 50 μl (1 000 copies/μl) for LOD and POD test, ⑤ a salmon sperm DNA sample with a volume of 50 μl (10 mg/ml; Invitrogen, Carlsbad, USA, CA 92008). The participants were requested to dilute the salmon sperm DNA samples to 20 ng/μl with ddH2O before use, ⑥ two bottles of 2×5 ml TaqMan Gene Expression Master Mix (Foster City, USA, CA 94404), and ⑦ three pairs of primers and probes in dry powder form, which were purchased from Sangon Biotech (Shanghai, China). The participants were requested to dilute the primers and probes to 10 μmol/L with ddH2O before use.
All packages were transported on ice to each participating laboratory by air freight. All participating laboratories received an operation protocol and a results report sheet. The participants were requested to operate strictly according to the operation protocol and to report any deviation from the protocol that may have occurred during the experimental operation.
For false-positive and false-negative rate evaluation, 12 blind samples (six positive and six negative samples) were evaluated for each method. The effective concentrations of positive and negative samples were 10 and 20 copies/reaction, respectively. The total number of reactions for each method was 144 among the 12 participating labs.
To evaluate the LODs and PODs, Microsoft Excel 2010 and a new mathematical statistical model[20] (Uhlig et al., 2015) were used, respectively. The pUC57-ghd plasmid DNA was serially diluted from 1 000 copies/μl to 4, 2, 1, 0.4, 0.2, 0.1, and 0.02 copies/μl using the salmon sperm DNA solution (20 ng/μl). Six replicates were performed for each dilution. The total number of reactions was 43 (including a blank control) for each method in each participating lab.
Results and Discussion
All 12 participating laboratories returned the experimental data (Ct values) in a timely manner. Although the participants were widely distributed in different countries, with different experimental operators, laboratory environments, and instrumentation, consistent experimental results were reported from all labs. None of the participating laboratories reported any experimental issues or obvious deviations for the sample preparation and detection procedures. In this collaborative study, six different real-time PCR cycler brands and/or models were used in the different participating laboratories, and the participants reported no abnormal results. Furthermore, in subsequent data analysis, no significant differences were identified, indicating that the detection method is robust and reliable.
Specificity
A comprehensive combination of theoretical (bioinformatics and sequence alignment) and practical tests was used to evaluate the specificity of the three developed methods. The theoretical specificities of the goat-, horse-, and donkey-specific primers and probes were evaluated by BLASTN similarity searches against the entire NCBI genome database. Sequence alignment results revealed no likelihood of significant cross-reaction with other species. Experimentally, exclusivity was evaluated using a broad range of DNA samples from 32 animal species, seven plant species, and one human.
For goat-material detection, only the expected positive signals were detected. For horse-material detection, only the expected positive signals were detected for horse, mule, hinny, Przewalskis horse, and zebra. For donkey-material detection, only the expected positive signals were detected from donkey, mule, hinny, and zebra (Table 2).
The inclusivities for the goat, horse, and donkey methods were tested on samples from varied breeding lines for each species, and the expected target amplification signals were detected for all breeds of the corresponding species (Table 3). These results confirm the specificity of the goat-, horse-, and donkey-specific detection methods.
False-positive and false-negative rates
For each detection method, the false-positive and false-negative rates were calculated based on the results of the 144 PCR reactions from the 12 participating laboratories. As expected, all positive samples were correctly identified as positive, all negative samples were confirmed as negative, and no abnormal results were reported. Thus, the false-positive and false-negative rates were determined to be 0% (Table 4) for all three methods, indicating the developed real-time PCR detection methods have excellent reliability.
LODs and PODs
In general, the LOD is defined as the lowest concentration at which 95% of replicates give positive qualitative results[21]. For each detection method, a total of 72 results were obtained from the 12 participating laboratories for each dilution level. Based on the collaborative ring trial results, the LOD95% for the goat, horse, and donkey detection was determined with a value of 5 copies/reaction (Table 5).
For POD determination, qualitative data from all PCR experiments at different dilutions were used to estimate the laboratory standard deviation (σL) and the theoretical median copy number. σL represents the relative between-laboratory variability at POD = 0.95. A value of 0.3 was obtained for the laboratory standard deviation of the detection methods, and the LOD95% (in copies) values of the theoretical median laboratory are 3.2, 3.2, and 3.1, respectively (Table 6). Thus, the LOD95% and POD evaluation results indicate that the goat-, horse-, and donkey-material detection methods have excellent sensitivities.
Conclusions
In this study, we developed species-specific real-time PCR methods for the detection of goat-, horse-, and donkey-derived material and organized a collaborative ring trial to validate these methods according to ISO 20813:2019[22]. This standardized methodology will assist in identifying fraudulent and/or mislabeled meat products. The study fully demonstrated the broad applicability and robustness of the methods. High specificities and low false positive/negative rates demonstrated the reliability and applicability of these real-time PCR detection methods, and LOD95% and POD analyses demonstrated their high sensitivities.
The three methods became parts 5, 6, and 7 of the ISO/TS 20224:2020 series standards[23-25] in July 2020 owing to their excellent and reliable performances and will now be widely used for the detection and identification of goat-, horse- and donkey-derived materials in meat products.
Acknowledgements
We are grateful to the following participants in this collaborative study: Caroline Araiz (Laboratoire Phytocontrol Parc Scientifique Georges BESSE II, France), Deyi Qiu (Inspection and Quarantine Technology Center of Zhongshan Entry-Exit Inspection and Quarantine Bureau, China), Eaqub Ali (Nanotechnology and Catalysis Research Center of Malaya University, Malaysia), Gang Wu (Oil Crops Research Institute, Chinese Academy of Agricultural Science, China), Inger Dagmar V lkel (Chemisches und Veterin runtersuchungsamt Westfalen A R Standort Arnsberg, Germany), Jiajian Xie (Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Inspection Test Center for Environmental Safety of Transgenic Crops and Plant Resistance, China), Mario Joao Gadanho (Biopremier of Innovation and Services in Biotechnology, Portugal), Qingping Zhang (Shanghai Institute of Quality Inspection and Technical Research, Food and Chemicals Quality Inspection Institute, China), Wenbing Chen (Inspection and Quarantine Technology Center of Fujian Entry-Exit Inspection and Quarantine Bureau, China), Wen Liang (Shanghai Institute of Measurement and Testing Technology, China), Wujun Jin (Biotechnology Research Institute, Chinese Academy of Agricultural Sciences; Inspection and Testing Center for Environmental Risk Assessment Genetically Modified Plant-related Microorganism, Ministry of Agriculture, China), and Xianzhong Feng (Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences, China).
References
[1] ALI ME, RAZZAK MA, HAMID SB, et al. Asing multiplex PCR assay for the detection of five meat species forbidden in Islamic foods[J]. Food Chem, 2015(177): 214-224.
[2] NAKYINSIGE K, MAN YB, SAZILI AQ. Halal authenticity issues in meat and meat products[J]. Meat Sci, 2012, 91(3): 207-214.
[3] HSIEH YH, OFORI JA. Detection of horse meat contamination in raw and heat-processed meat products[J]. J Agric Food Chem, 2014, 62(52): 12536-12544.
[4] NAU JY. Horse meat: first lessons of a scandal[J]. Rev Med Suisse, 2013, 9(376): 532-533.
[5] FLOREN C, WIEDEMANN I, BRENIG B, et al. Species identification and quantification in meat and meat products using droplet digital PCR (ddPCR)[J]. Food Chem, 2015(173): 1054-1058.
[6] CAI Y, WANG Q, HE Y, et al. Interlaboratory validation of a real-time PCR detection method for bovine- and ovine-derived material[J]. Meat Sci, 2017(134): 119-123.
[7] CHENG X, HE W, HUANG F, et al. Multiplex real-time PCR for the identification and quantification of DNA from duck, pig and chicken in Chinese blood curds[J]. Food Research International, 2014(60): 30-37.
[8] YUSOP MHM, MUSTAFA S, CHE MAN YB, et al. Detection of raw pork targeting porcine-specific mitochondrial cytochrome B gene by molecular beacon probe real-time polymerase chain reaction[J]. Food Analytical Methods, 2012, 5(3): 422-429.
[9] KESMEN Z, YETIMAN AE, SAHIN F, et al. Detection of chicken and turkey meat in meat mixtures by using real-time PCR assays[J]. J Food Sci, 2012, 77(2): 167-173.
[10] ALI ME, HASHIM U, MUSTAFA S, et al. Analysis of pork adulteration in commercial meatballs targeting porcine-specific mitochondrial cytochrome b gene by TaqMan probe real-time polymerase chain reaction[J]. Meat Science, 2012, 91(4): 454-459.
[11] DRUML B, MAYER W, CICHNA-MARKL M, et al. Development and validation of a TaqMan real-time PCR assay for the identification and quantification of roe deer (Capreolus capreolus) in food to detect food adulteration[J]. Food Chem, 2015(178): 319-326.
[12] FANG X, ZHANG C. Detection of adulterated murine components in meat products by TaqMan(c) real-time PCR[J]. Food Chem, 2016(192): 485-490.
[13] HOSSAIN MAM, ALI ME, SULTANA S, et al. Quantitative Tetraplex real-time polymerase chain reaction assay with TaqMan probes discriminates cattle, buffalo, and porcine materials in food chain[J]. J Agric Food Chem, 2017, 65(19): 3975-3985.
[14] KESMEN Z, GULLUCE A, SAHIN F, et al. Identification of meat species by TaqMan-based real-time PCR assay[J]. Meat Science, 2009, 82(4): 444-449.
[15] ISO 21571: 2005. Food stuffs—methods of analysis for the detection of genetically modified organisms and derived products—nucleic acid extraction. A.1: preparation of PCR-quality DNA using phenol/chloroform-based DNA extraction methods.1-43.
[16] WANG Q, CAI Y, HE Y, et al. Droplet digital PCR (ddPCR) method for the detection and quantification of goat and sheep derivatives in commercial meat products[J]. European Food Research and Technology, 2018, 244(4): 767-774.
[17] WADE CM, GIULOTTO E, SIGURDSSON S, et al. Genome sequence, comparative analysis, and population genetics of the domestic horse[J]. Science, 2009, 326(5954): 865-867.
[18] HUANG J, ZHAO Y, BAI D, et al. Donkey genome and insight into the imprinting of fast karyotype evolution[J]. Sci Rep, 2015(5): 14106.
[19] DENG YM, SPIRASON N, IANNELLO P, et al. A simplified Sanger sequencing method for full genome sequencing of multiple subtypes of human influenza A viruses[J]. J Clin Virol, 2015(68): 43-48.
[20] UHLIG S, FROST K, COLSON B, et al. Validation of qualitative PCR methods on the basis of mathematical-statistical modelling of the probability of detection[J]. Accreditation and Quality Assurance, 2015, 20(2): 75-83.
[21] GROHMANN L, BRUNEN-NIEWELER C, NEMETH A, et al. Collaborative trial validation studies of real-time PCR-based GMO screening methods for detection of the bar gene and the ctp2-cp4epsps construct[J]. J Agric Food Chem, 2009, 57(19): 8913-8920.
[22] ISO 20813:2019. Molecular biomarker analysis—Methods of analysis for the detection and identification of animal species in foods and food products (nucleic acid-based methods) —General requirements and definitions. 1-27.
[23] ISO/TS 20224-5:2020. Molecular biomarker analysis—Detection of animal-derived materials in foodstuffs and feedstuffs by real-time PCR—Part 5: Goat DNA detection method.1-14.
[24] ISO/TS 20224-6:2020. Molecular biomarker analysis—Detection of animal-derived materials in foodstuffs and feedstuffs by real-time PCR—Part 6: Horse DNA detection method. 1-15.
[25] ISO/TS 20224-7:2020. Molecular biomarker analysis—Detection of animal-derived materials in foodstuffs and feedstuffs by real-time PCR—Part 7: Donkey DNA detection method. 1-14.Editor: Yingzhi GUANG Proofreader: Xinxiu ZHU
